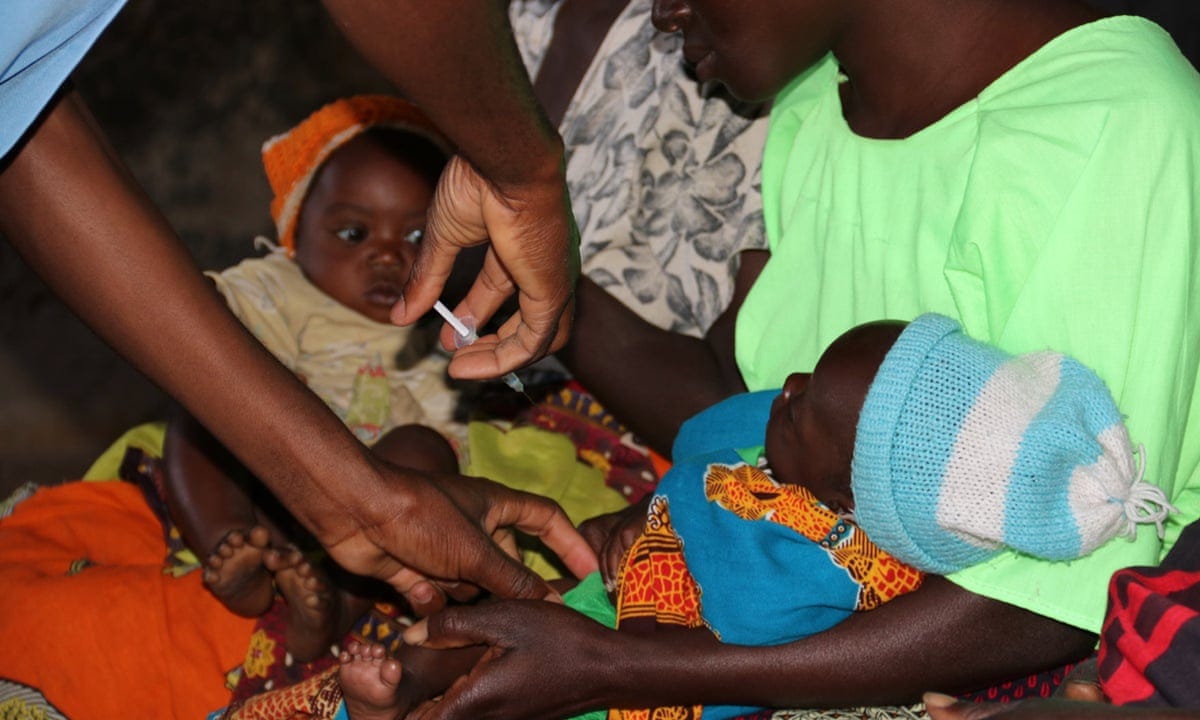
Over 1 million African children protected by first malaria vaccine
Thanks to a pilot program coordinated by WHO, over 1 million children in Ghana, Kenya, and Malawi have now received one or more doses of the world's first malaria vaccine.
MALAWI: Thanks to a pilot program coordinated by WHO, over 1 million children in Ghana, Kenya, and Malawi have now received one or more doses of the world's first malaria vaccine. The malaria vaccine pilots, which were first launched by the Malawian government in April 2019, have demonstrated that the RTS,S/AS01 (RTS,S) vaccine is safe and feasible to administer and that it significantly reduces deadly severe malaria.
These findings paved the way for the historic WHO recommendation in October 2021 to expand the use of RTS,S among children living in areas with moderate to high malaria transmission. WHO estimates that if widely used, the vaccine could save the lives of an additional 40 000 to 80 000 African children each year.
Gavi, the Vaccine Alliance, has committed more than US$ 155 million to support the introduction, procurement, and delivery of the malaria vaccine in Gavi-eligible countries in Sub-Saharan Africa. Countries can get advice from WHO on whether and how to use RTS,S as an additional tool to reduce malaria illness and deaths in children.
“As a malaria researcher in my early career, I dreamed of the day we would have an effective vaccine against this devastating disease,” said Dr Tedros Adhanom Ghebreyesus, WHO Director-General.
“This vaccine is not just a scientific breakthrough, it’s life-changing for families across Africa. It demonstrates the power of science and innovation for health. Even so, there is an urgent need to develop more and better tools to save lives and drive progress towards a malaria-free world.”
Prospects for new interventions
RTS,S is a first-generation vaccine that may be supplemented in the future by other vaccines with comparable or greater efficacy. The World Health Organization applauds progress in the development of R21/Matrix-M and other malaria vaccine candidates in early clinical trials. The completion of clinical trials for these vaccines will be critical in determining their safety and efficacy profiles. WHO also applauds BioNTech, the manufacturer of the Pfizer-BioNTech COVID-19 vaccine, for announcing plans to develop a malaria vaccine using mRNA technology.
A number of new vector control tools and technologies have been submitted to WHO for evaluation. If they prove effective in controlling the disease, WHO will develop new recommendations or revise existing ones to support their use. New insecticide-treated nets, spatial mosquito repellents, gene-drive approaches, and sugar baits designed to attract and kill Anopheles mosquitoes are among these.
There are also new medications in the works. WHO applauds the Australian Therapeutic Goods Administration's recent approval of dispersible tablets of single-dose tafenoquine for the prevention of P. vivax malaria in children. The US Food and Drug Administration and drug regulatory bodies in other countries, including Brazil, Peru, and Thailand, have also approved tafenoquine for use in adults. Tafenoquine, as a single dose, is expected to improve patient adherence to treatment. The current standard of care calls for a seven- to fourteen-day course of medication.
Other antimalarial medicines with novel modes of action are being developed for the treatment of both simple and severe malaria. Ganaplacide-Lumefantrine, which is currently in Phase II clinical trials, is the first non-artemisinin combination therapy and could be useful in the fight against drug-resistant malaria in Africa.
Aside from drug resistance, WHO has reported other pressing threats in the fight against malaria, such as insecticide-resistant mosquitos, an invasive malaria vector that thrives in both urban and rural areas, and the emergence and spread of mutated P. falciparum parasites, which are undermining the effectiveness of rapid diagnostic tests. To contain these threats, innovation in tools and strategies, as well as a more strategic use of the tools that are currently available, will be critical.
More investment needed
According to the 2021 World Malaria Report, global progress in reducing malaria cases and deaths has slowed or stalled in recent years, particularly in the disease's most affected countries. The report emphasizes the importance of continued innovation in the research and development of new tools if the world is to meet the WHO malaria strategy's 2030 targets.
In 2020, funding for malaria-related research and development will have surpassed US$ 619 million. In the period 2021–2030, an average annual R&D investment of $851 million will be required.
Making better use of the tools we have now
Reaching global malaria targets will also necessitate changes in how currently available tools are used. The "High burden to high impact" approach, launched by WHO and the RBM Partnership to End Malaria in 2018, has been collecting and analyzing malaria data to better understand the disease's geographical spread.
Instead of taking the same approach to malaria control everywhere, they are considering the potential impact of tailored intervention packages informed by local data and disease context. These analyses will help countries use available funds more effectively, efficiently, and equitably.